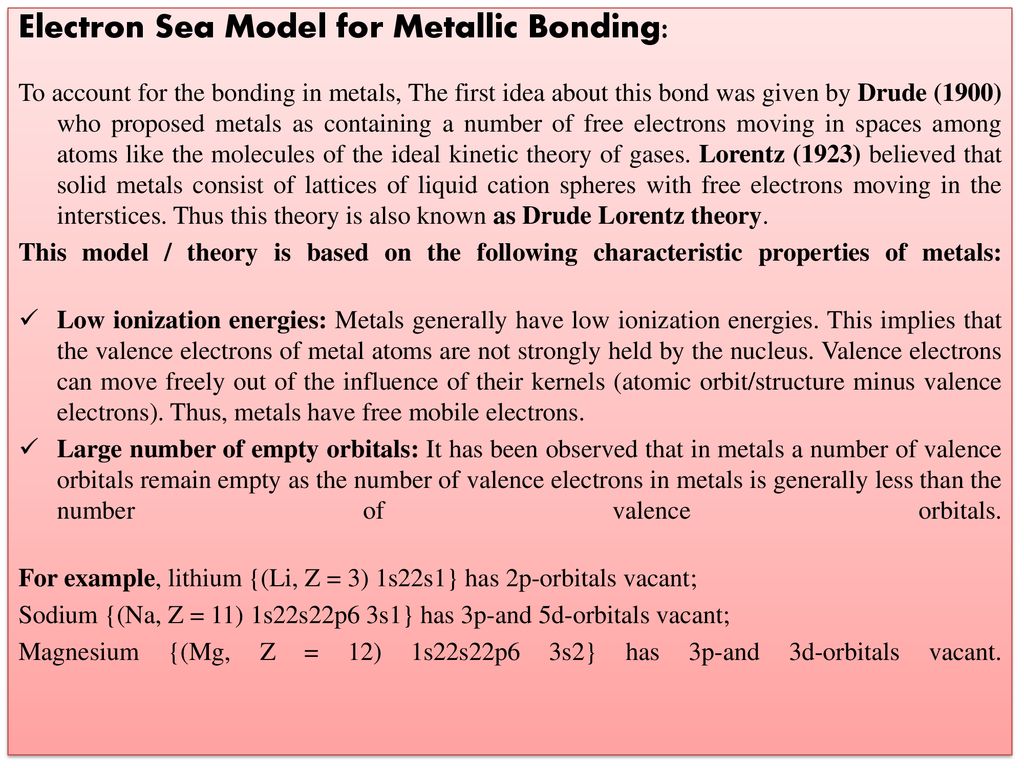
Describe the Electron Sea Model of Metallic Bonding
Sodium has one valence electron. Describe the electron-sea model of metallic bonding.
This free movement of delocalized.
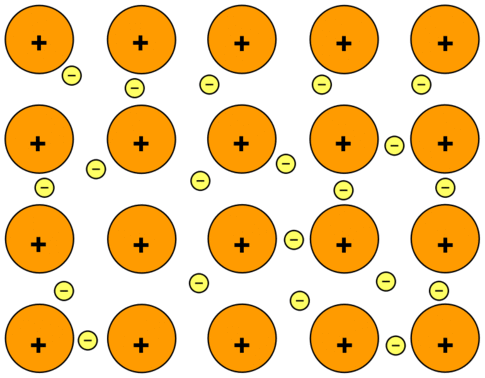
. Describe the electron sea model of metallic bonding LIMITED TIME OFFER. And the positive sodium atom immersed into the sea of delocalized electrons. A metallic bond is a compound of opposite charges.
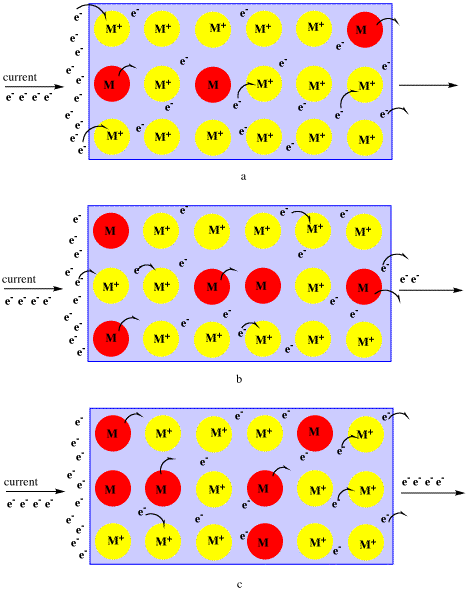
Describe a metallic bond. Metals are good conductors of electricity because the electrons in the electron sea are free to flow and carry electric current. Johannesson I II III IV Multiple Covalent Bonds Single bond.
This is why this model is known as the electron sea model. Positive atomic nuclei surrounded by a sea of delocalized electrons the blue dots. The metal is held together by the attraction between the metal ___ and the sea of electrons.
The atoms are relatively fixed in. Give at least one similarity and one difference between the models. View METALLIC BONDING OBJECTIVEdocx from SCIENCE Chemistry at River Dell Regional H S.
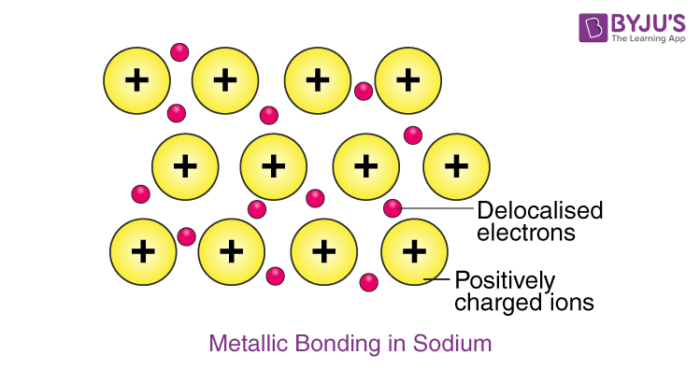
The electrons present in the outer energy levels of the bonding metallic atoms are not held by any specific and can move easily from one atom to the next. The Electron Sea Model. Metallic bond is the electrostatic force between the positively charged metallic ions and the sea of electrons.
Electrons all have approximately the same energy. How is the electron sea model of metallic bonding different from the band theory. Metals are ductile and malleable.
This electron can delocalize through out the metal crystal to form the metallic bond. Metallic bonding may be described as the sharing of free electrons among a lattice of positively charged metal ionsThe structure of metallic bonds is very different from that of covalent and ionic bondsWhile ionic bonds join metals to nonmetals and covalent bonds join nonmetals to nonmetals metallic bonds are responsible for the bonding between metal atoms. What is the electron sea model.
Select all the statements that correctly describe the electron-sea model of metallic bonding. Metallic bonding is known as the electron-sea model. Posted on March 4 2019.
Using the electron sea model to explain it. Each atom contributes its valence electrons to a region surrounding the atoms. The Electron-Sea Model Why Metals Are AP Chemistry- Practice Bonding Questions for ExamDifference Between Ionic Covalent and Metallic bonds with Quiz Worksheet - Ionic Compound Formation Properties 42 Classifying Chemical Reactions Chemistryquiz 4.
That is to say instead of orbiting their respective metal atoms they form a sea of electrons that surrounds the positively charged atomic nuclei of the interacting metal ions. In terms of this model all the metal atoms contribute their ___ electrons to form an electron sea so that the electrons are ___ or shared among all the atoms. This model proposes that all the metal atoms in a metallic solid contribute their valence electrons to form a sea of electron.
Metallic bonding is an attraction between positively charged metal ions and a sea of surrounding negatively charged electrons. The model of metallic bonding where electrons float free in a sea of electrons around metal atoms. How are they the same.
It is generally viewed as the result of mutual sharing of many electrons by atoms. Learn about metallic bonding with an explanation of the unique properties of metals and understand why metals are good electrical conductors. Describe the electron-sea model of metallic bonding.
The atoms are arranged so that each sodium atom is surrounded by eight other sodium atoms. GET 20 OFF GRADE YEARLY SUBSCRIPTION. In a polar covalent bond the electron density is highest over the more electronegative atom.
These valence electrons hold the positive ions together throughout the structure of the metal. In metallic bonds the valence electrons from the s and p orbitals of the interacting metal atoms delocalize. The model shows a portion of the crystal structure of solid sodium.
Aluminium has 3 valence electrons each of the aluminium atom will release the 3. These electrons are then free to move about the mostly vacant outer orbits of all metal atoms. Electrons move among orbitals of different energies.
View ch-6-sect_3-5_notesppt from CHEM INORGANIC at University of Texas Brownsville. The characteristics of metallic bonds explain a number of the unique properties of metals. The valence electrons of the.
This model proposes that all the metal atoms in a metallic solid contribute their valence electrons to form a sea of electrons. Metallic bonds are characterized by the overlap of outer orbitals where electrons are allowed to move freely from atom to atom in the sea of electron model. Up to 256 cash back Get the detailed answer.
Find step-by-step Chemistry solutions and your answer to the following textbook question. The simplest model used to describe the bonding in metals is the electron-sea model. The positive ions are immersed into the sea of electrons.
Describe the electron sea model of metallic bonding OneClass.

Metallic Bonding 7 3 Electron Sea Model The Electron Sea Model Proposes That All The Metal Atoms In A Metallic Solid Contribute Their Valence Electrons Ppt Download
Title Metallic Bond Dr Ppt Download

Metallic Bonding Definition And Properties

Section 7 4 Section 7 4 Metallic Bonds And The Properties Of Metals Describe A Metallic Bond Relate The Electron Sea Model To The Physical Properties Ppt Download
Metallic Bonds Properties Examples Explanation Of Metallic Bonds

Metallic Bonding Chemical Bonding Metallic Bonding Objectives Describe The Electron Sea Model Of Metallic Bonding And Explain Why Metals Are Good Electrical Ppt Download
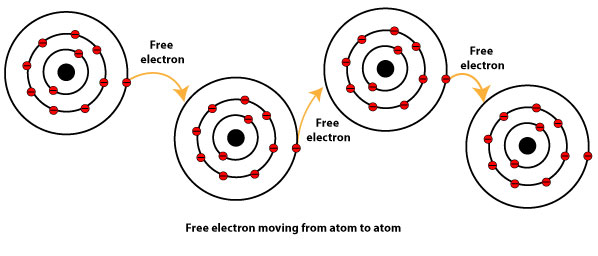
What Is Metallic Bonding Using The Electron Sea Model To Explain It

Metallic Bonding 7 3 Electron Sea Model The Electron Sea Model Proposes That All The Metal Atoms In A Metallic Solid Contribute Their Valence Electrons Ppt Download

Bonding In Metals The Electron Sea Model Introduction To Chemistry

Electron Sea Model Easy Science Electron Sea Model Electrons Easy Science
What Are Some Ways To Describe Metallic Bonding Quora
Properties Of Metallic Bonding Ppt Download

Draw A Neat Diagram Of Electron Sea Model Of Metallic Bonding And Label The Kernals And Valence Brainly In

Metallic Bond Study Guide Inspirit
Metallic Bonding Properties Tutorial Now With Animations The Crash Chemistry Academy Youtube

Learn About Electron Sea Model Chegg Com

Metallic Bonding And The Electron Sea Model Electrical Conductivity Basic Introduction Youtube

Metallic Bonding Chemical Bonding Metallic Bonding Objectives Describe The Electron Sea Model Of Metallic Bonding And Explain Why Metals Are Good Electrical Ppt Download

Comments
Post a Comment